Image 1 of 5
Image 1 of 5

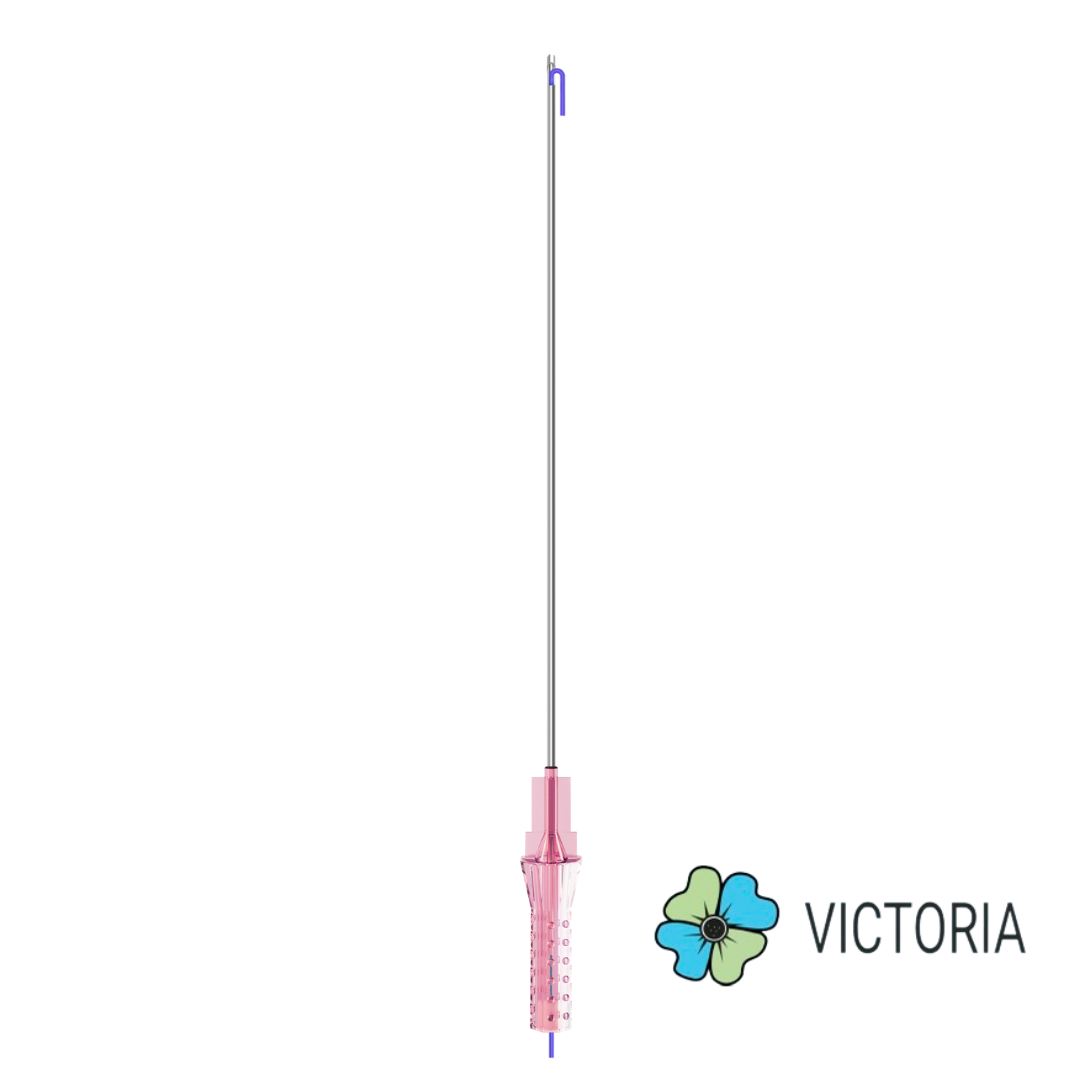 Image 2 of 5
Image 2 of 5

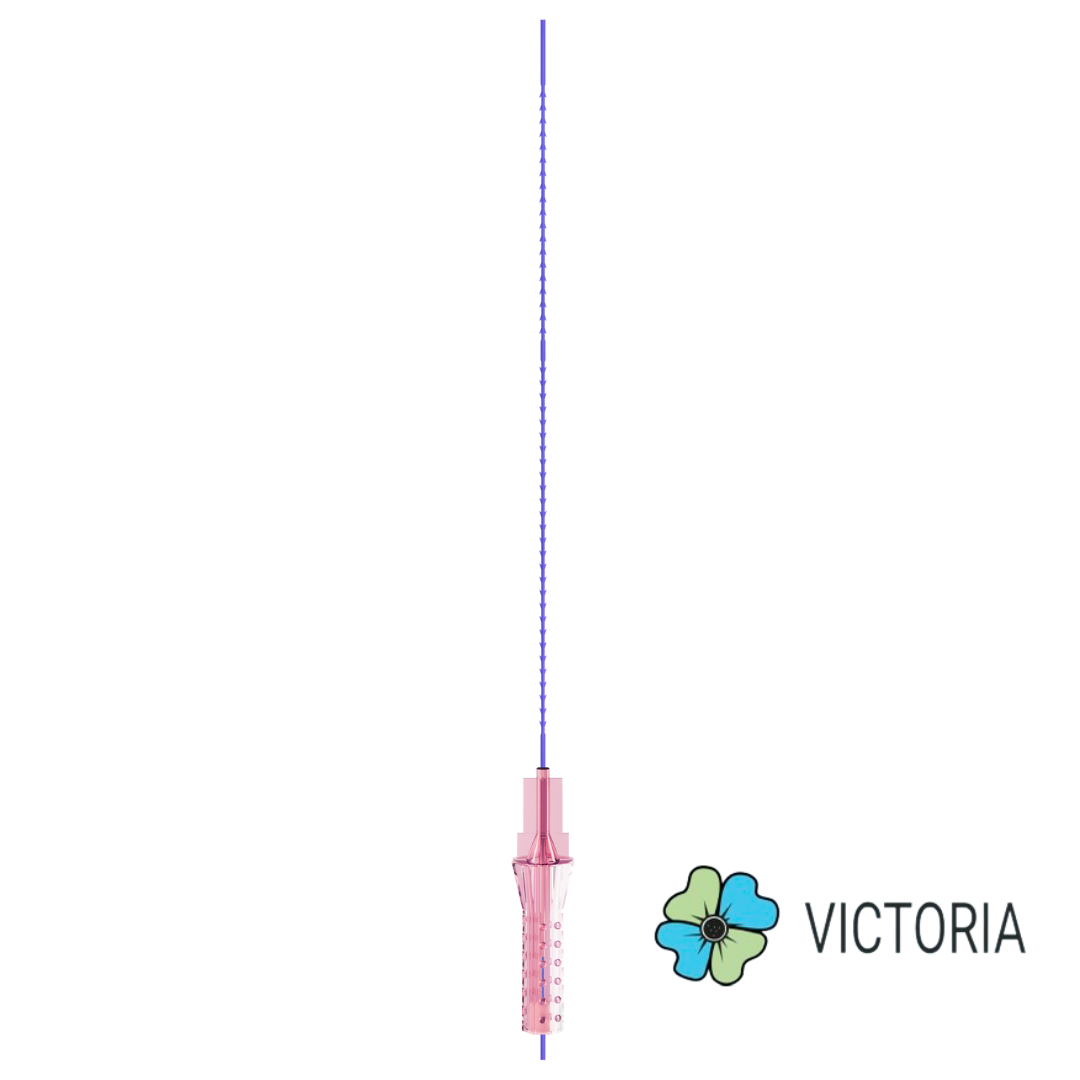 Image 3 of 5
Image 3 of 5

 Image 4 of 5
Image 4 of 5

 Image 5 of 5
Image 5 of 5






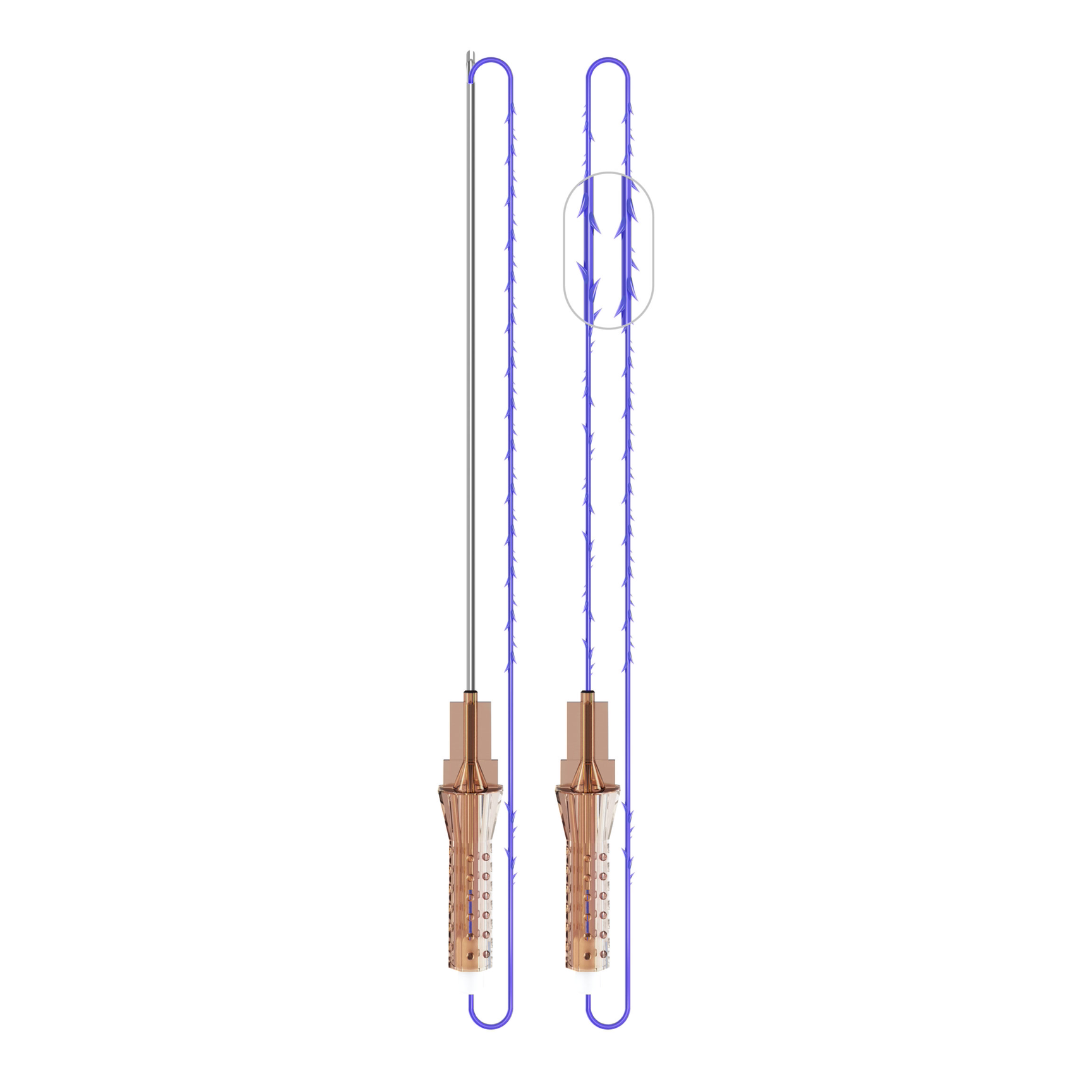
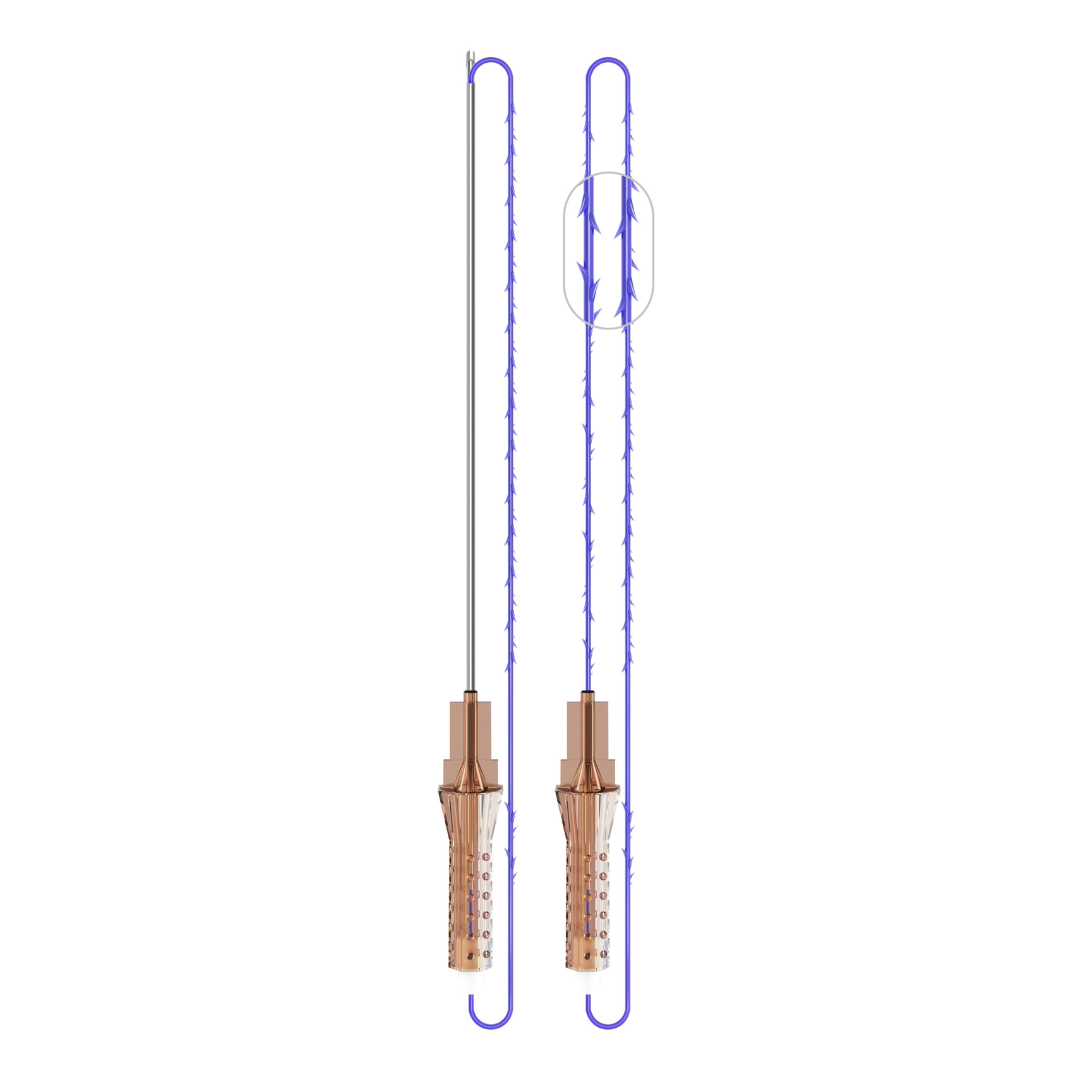
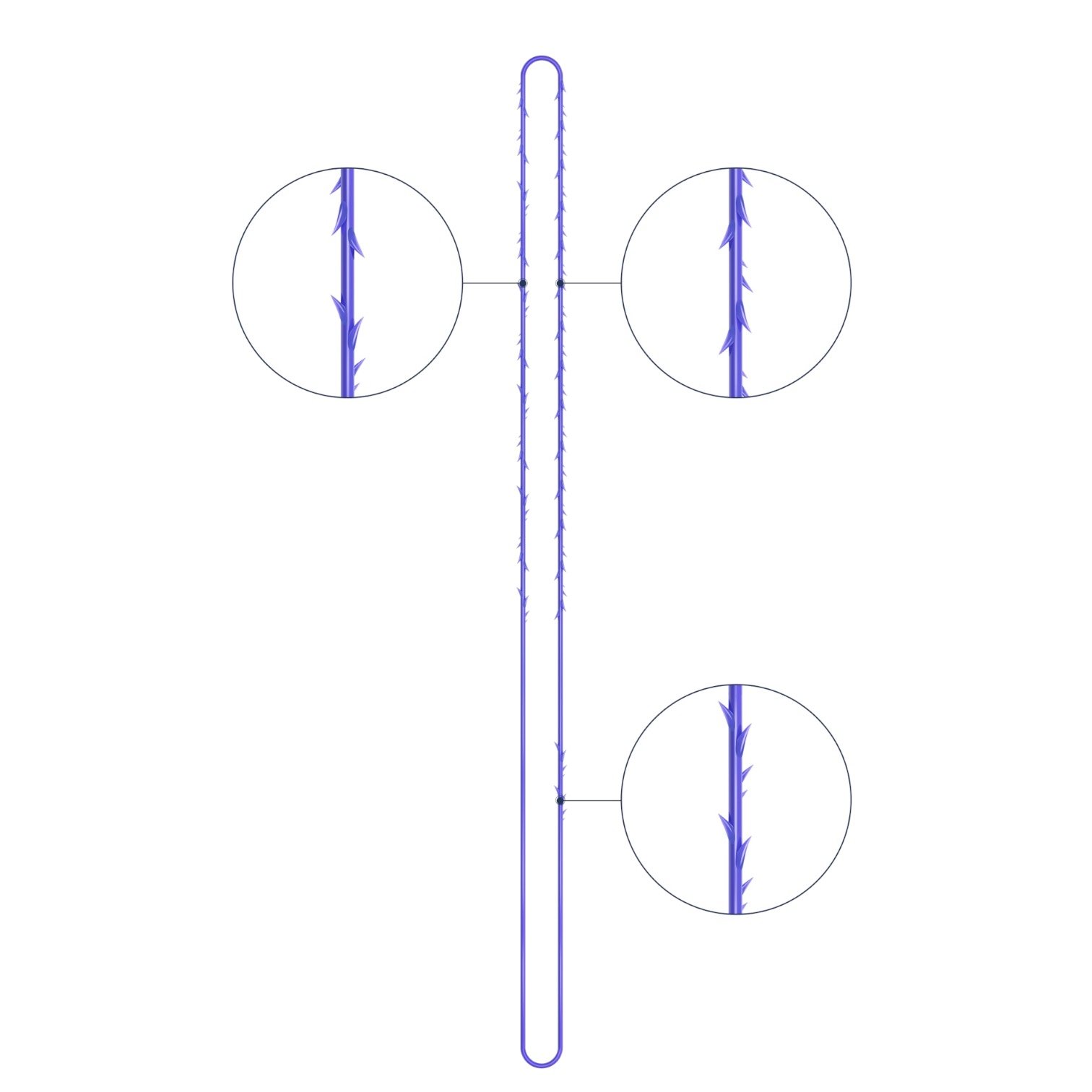
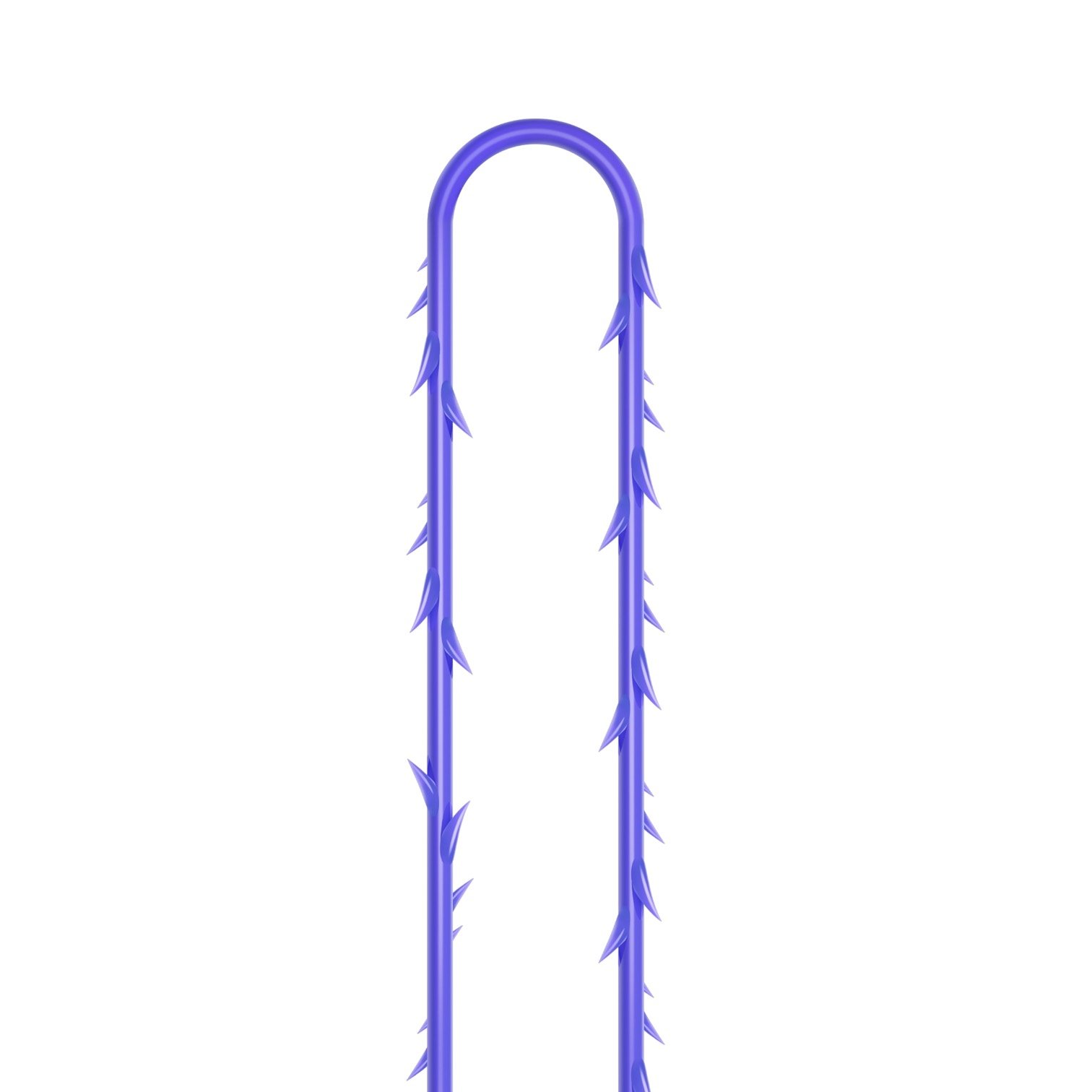
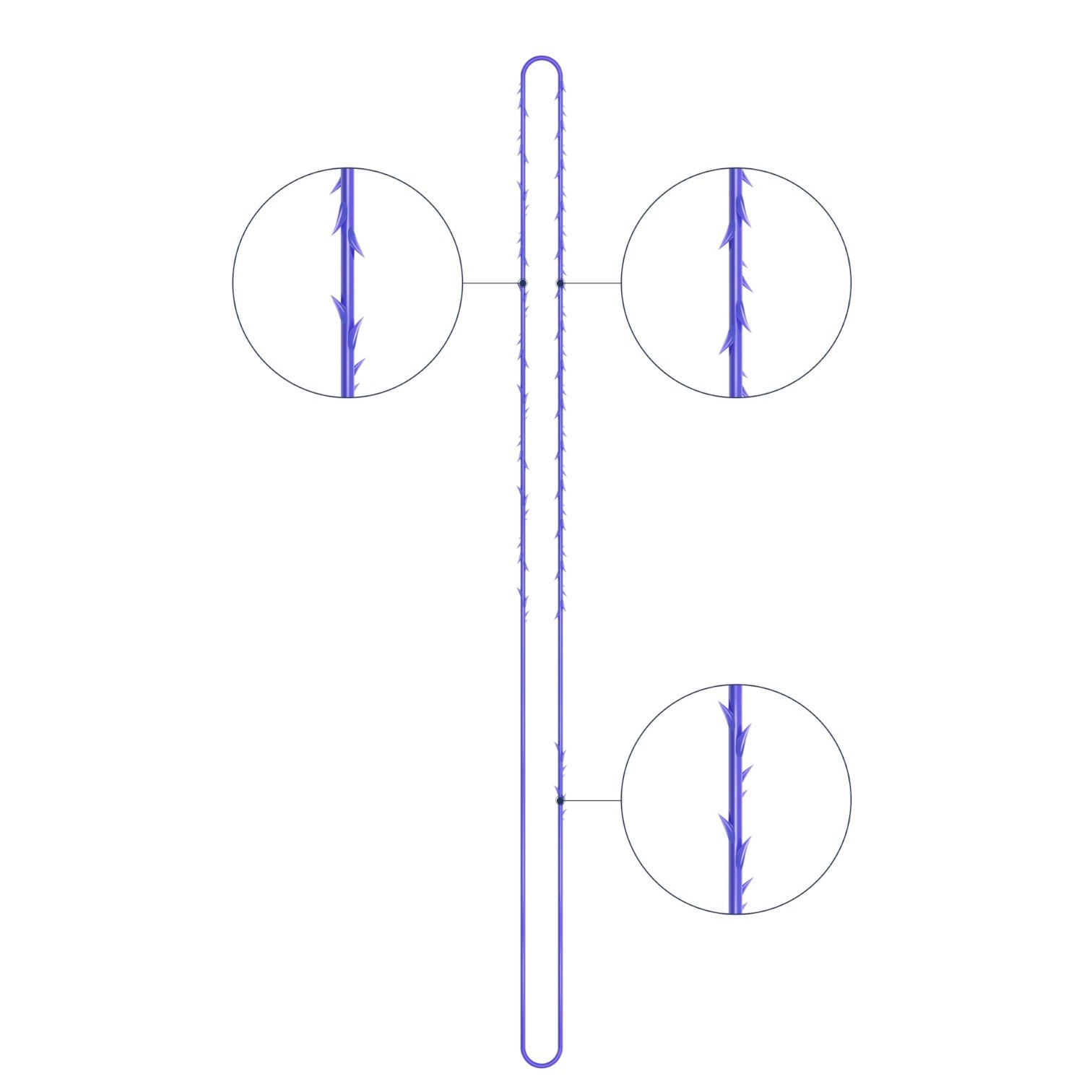
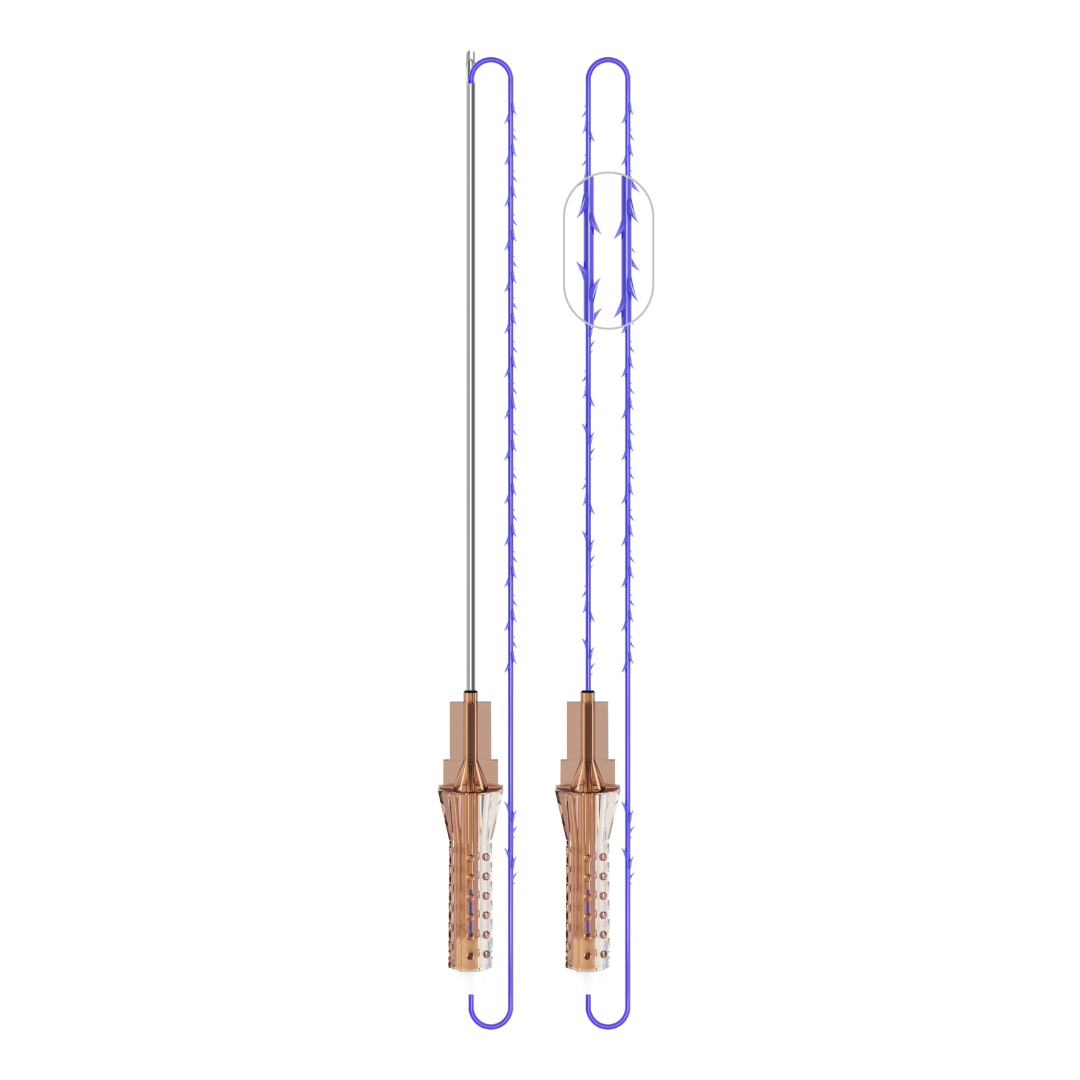
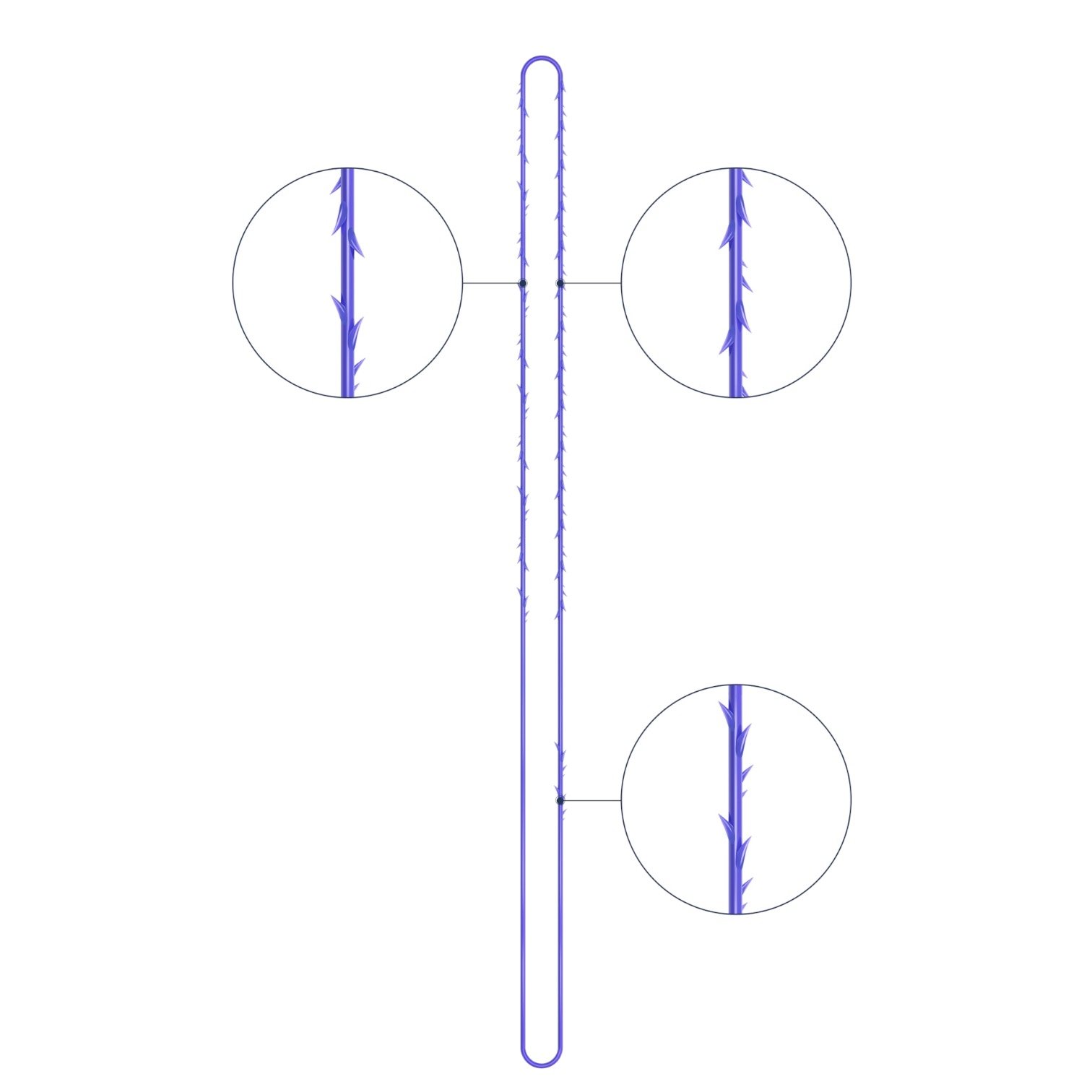
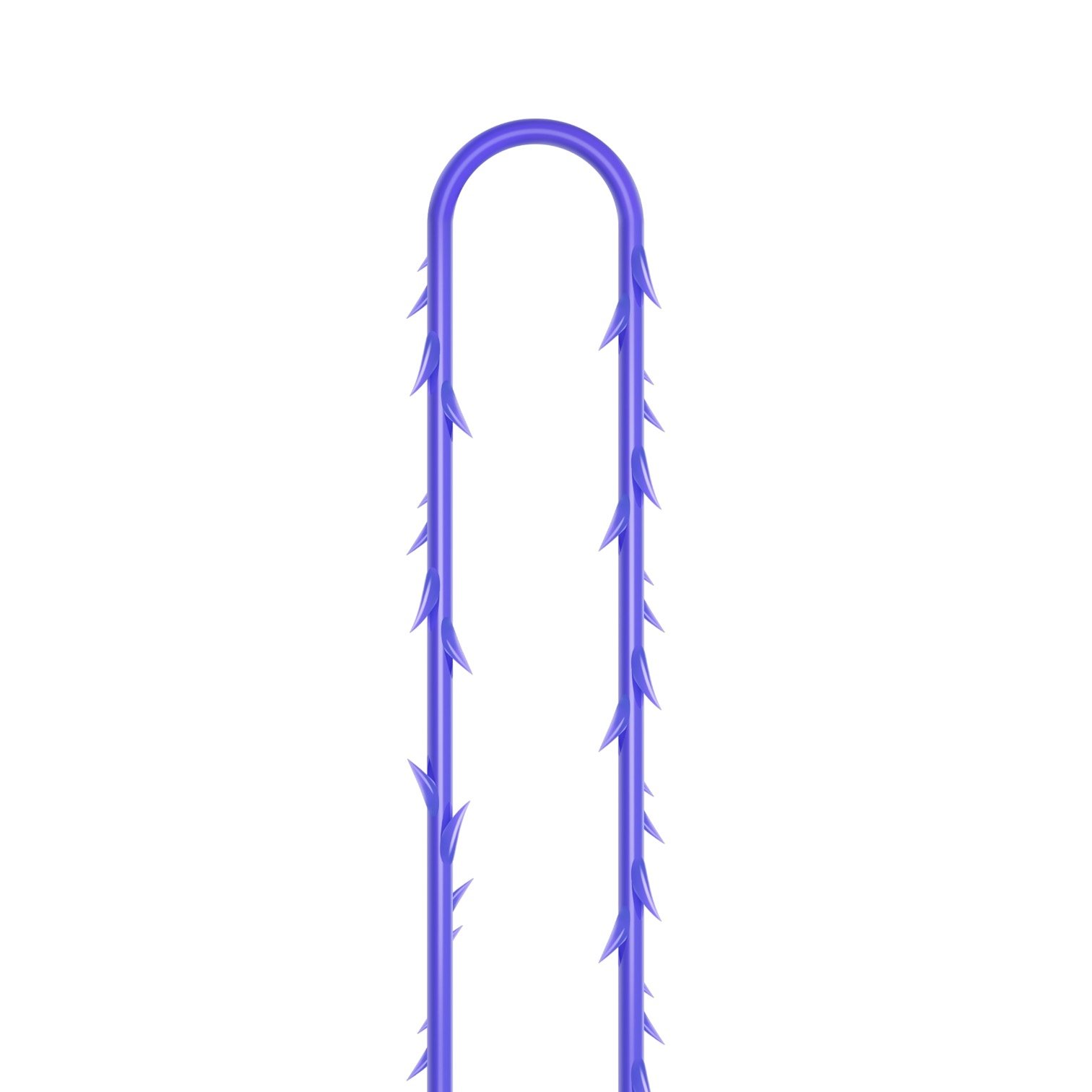
VICTORIA PDO MOLDED COG Threads 18G100 185mm
Suture type: bi-directional molded thread Needle caliber:18G100 (cannula W) Suture’s length: 185 mm Suture’s USP: 1-0
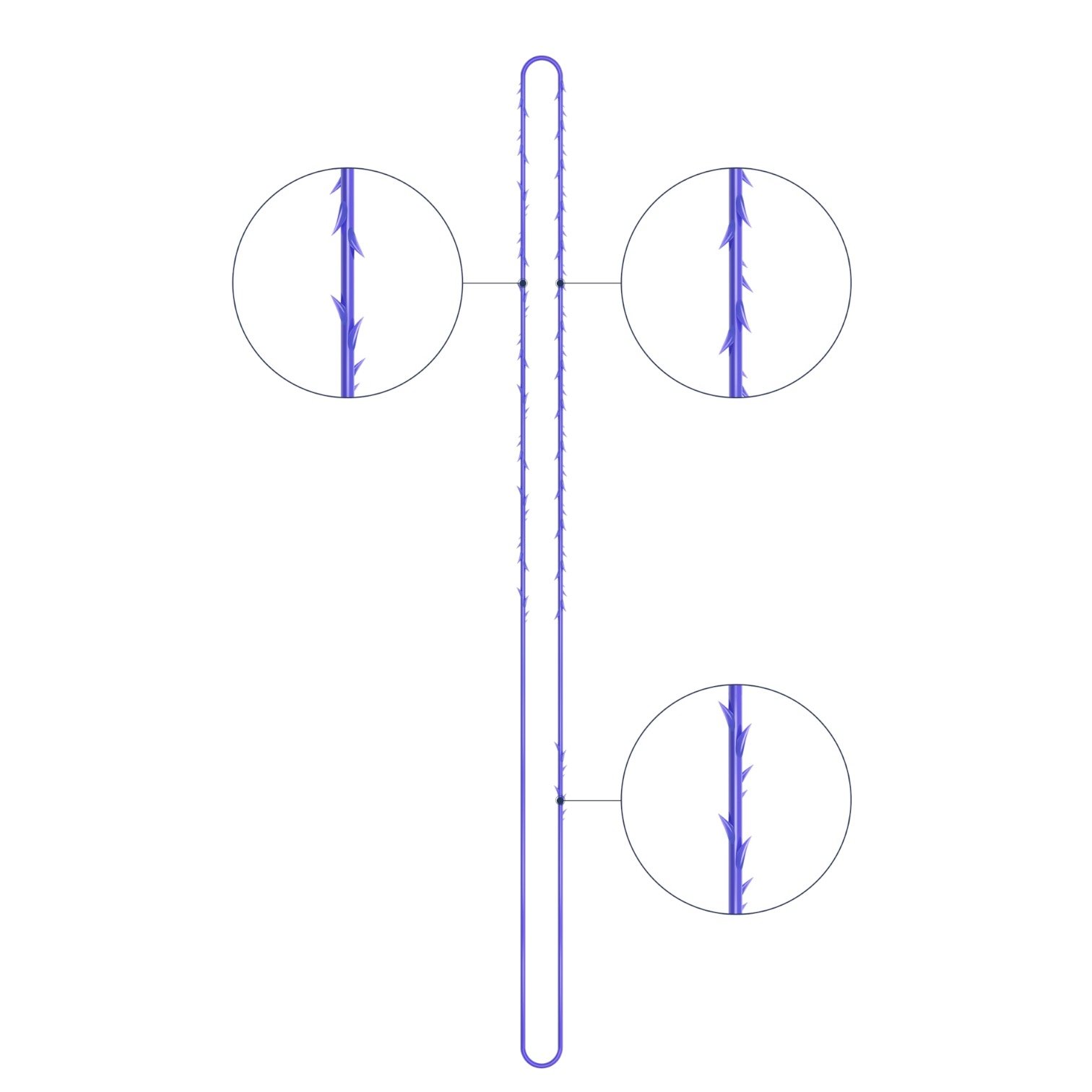
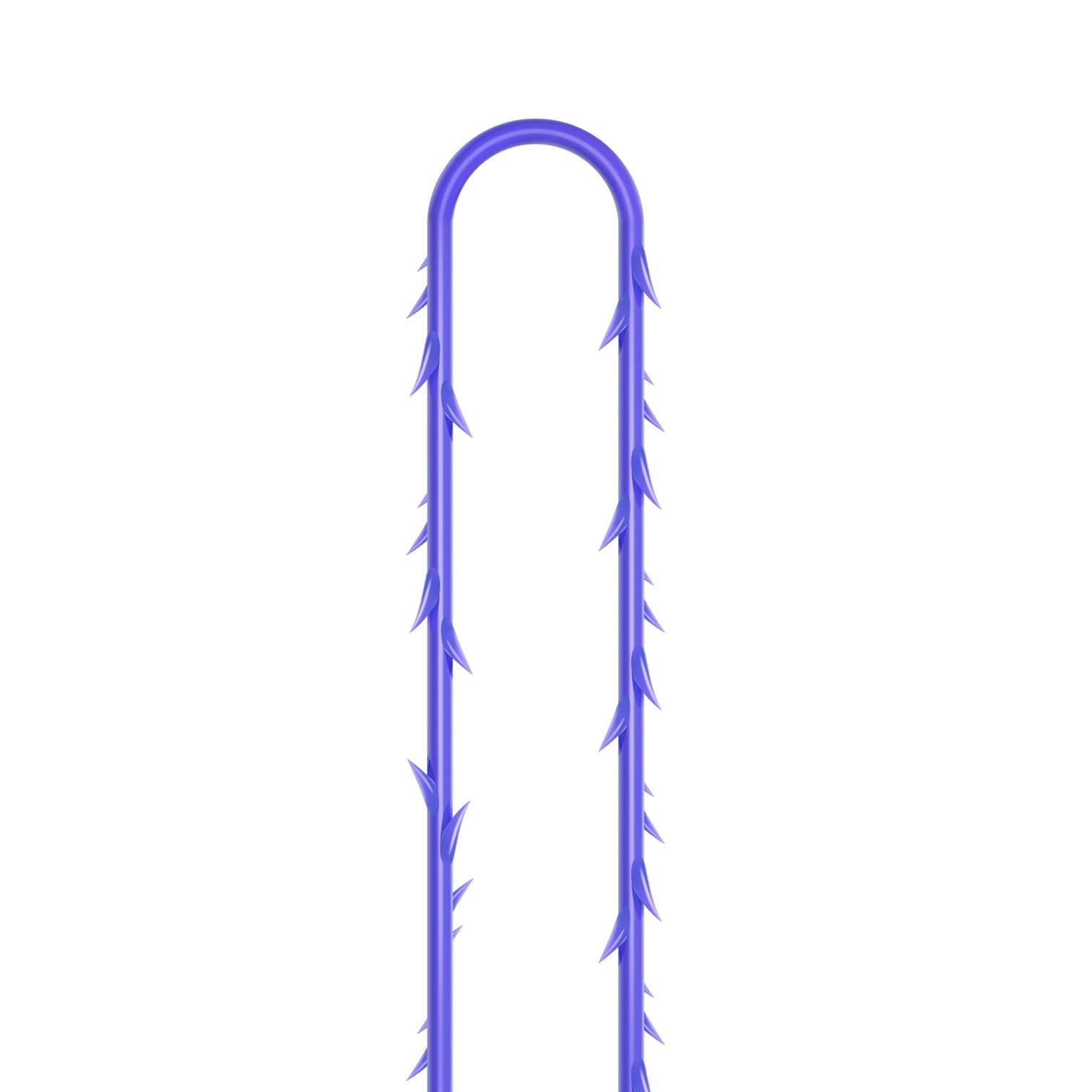
The VICTORIA MOLDED IRIS PDO threads are created using a unique patented embossing technology that significantly strengthens the PDO thread, making it exceptionally durable and resistant to breakage. Specially shaped protrusions formed by pressing provide excellent adhesion to the skin tissue and securely hold it in place
Package: 1 Pack for 8 pcs
FDA Approved
Made in South Korea
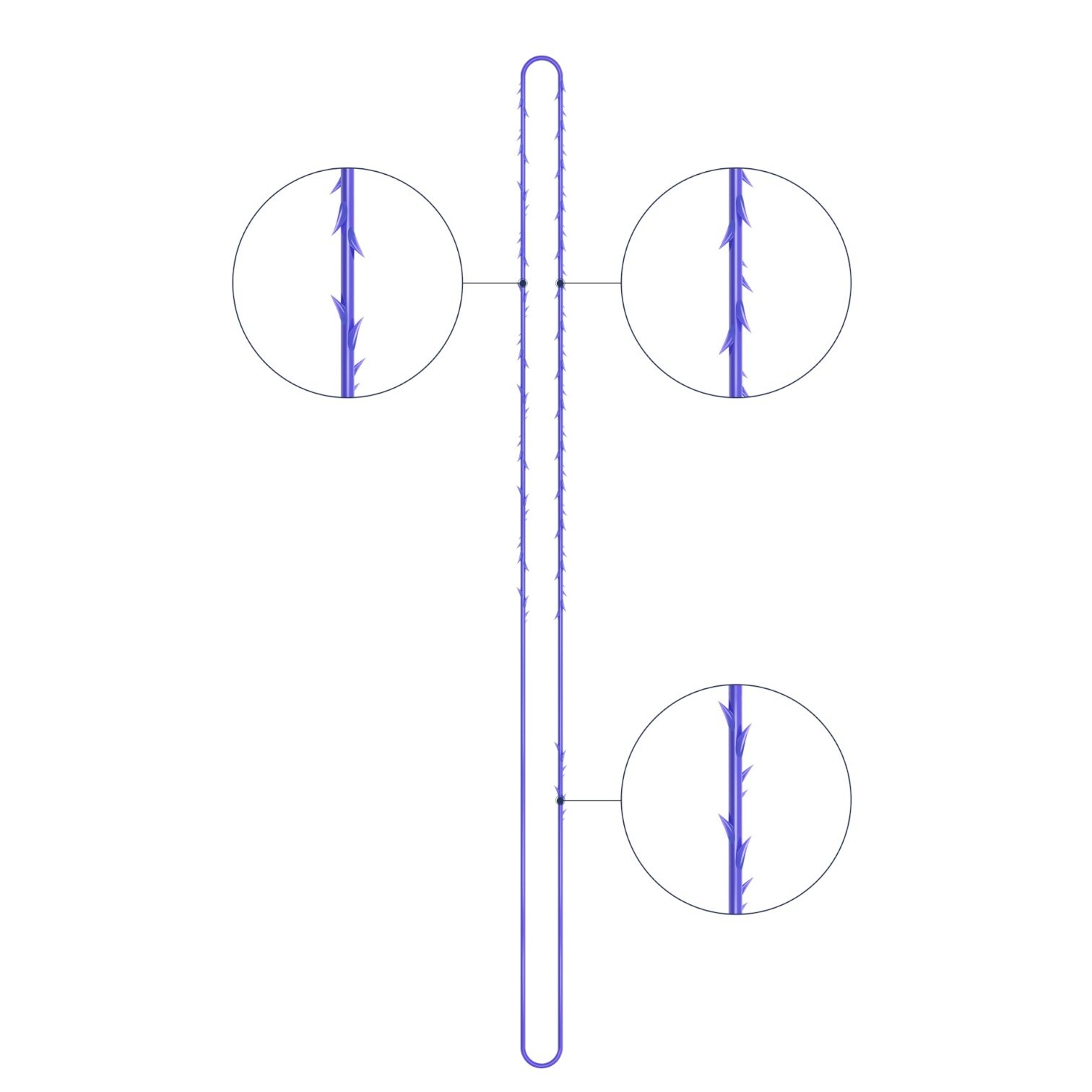
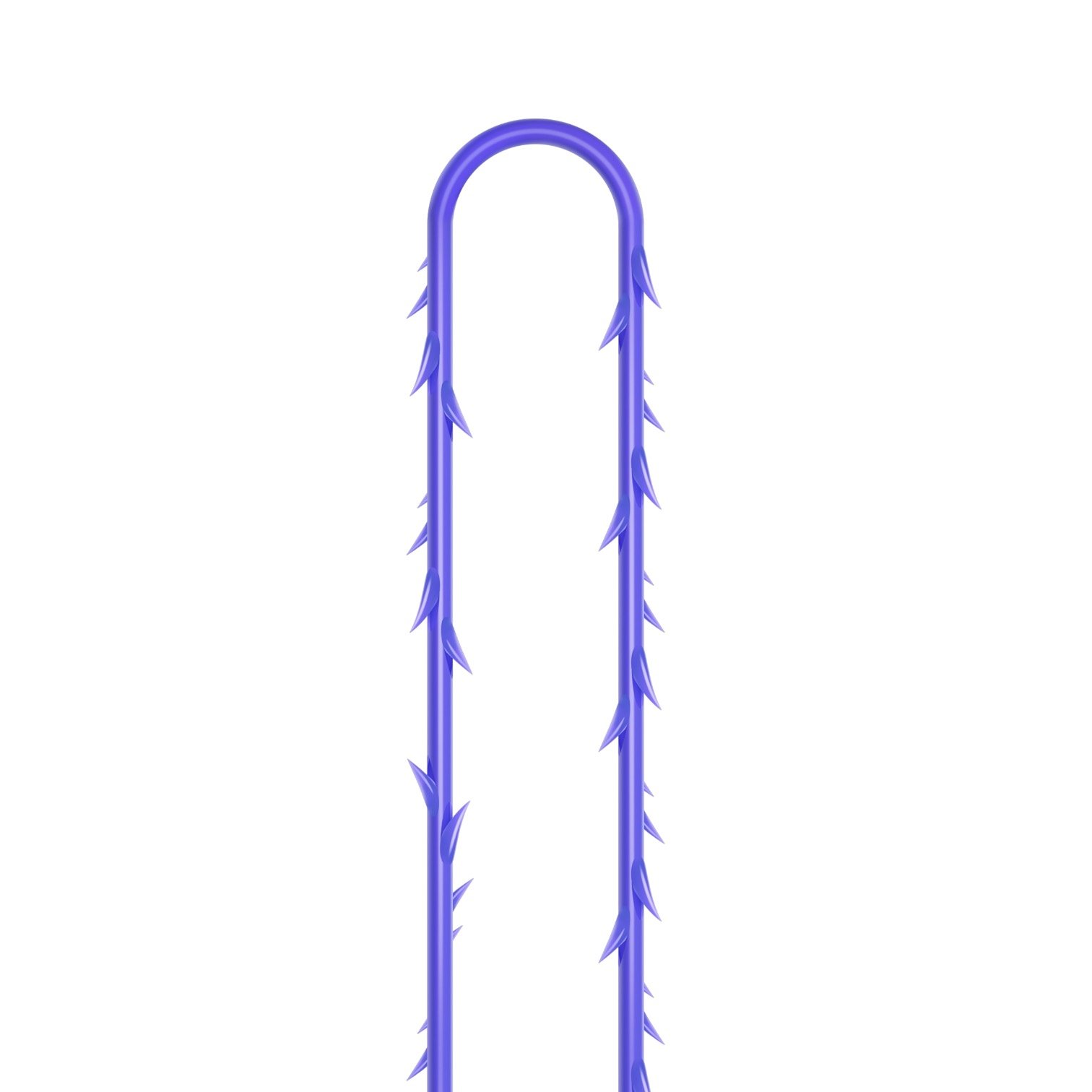
Suture type: bi-directional molded thread Needle caliber:18G100 (cannula W) Suture’s length: 185 mm Suture’s USP: 1-0
The VICTORIA MOLDED IRIS PDO threads are created using a unique patented embossing technology that significantly strengthens the PDO thread, making it exceptionally durable and resistant to breakage. Specially shaped protrusions formed by pressing provide excellent adhesion to the skin tissue and securely hold it in place
Package: 1 Pack for 8 pcs
FDA Approved
Made in South Korea
VICTORIA PDO Threads: Advanced Biodegradable Technology
VICTORIA PDO threads are meticulously crafted from 100% fine-porous and high-density polydioxanone, ensuring optimal water content of 100 ppm within the polymer matrix. This innovative material composition features a predominance of crystalline structural nodes over amorphous regions, which protects against rapid resorption while maintaining maximum tensile strength. This characteristic effectively facilitates mechanotransduction effects, crucial for tissue integration

VICTORIA PDO Threads: Exceptional Strength and Integration
Due to their unique formulation, VICTORIA PDO threads exhibit remarkable strength and resistance to rupture both prior to and after implantation. Once implanted, these lifting threads integrate seamlessly into the dermal layers, ensuring stability and effectiveness.
VICTORIA PDO Threads: Biodegradation and Safety
The high density of polydioxanone, combined with the absence of micro-cracks both on the surface and within the polymer, prevents the threads from absorbing excess fluid. This feature minimises the risk of fragmentation or disintegration during the biodegradation process, significantly reducing the potential for thread rupture within the tissues. Furthermore, VICTORIA PDO threads are designed for uniform and predictable bioresorption, aligning perfectly with the rate of healing tissue formation. This results in a more durable and long-lasting aesthetic outcome from the thread lifting procedure.
VICTORIA PDO Threads: Longevity of Physical Properties
In the initial 90 days following implantation, the VICTORIA PDO Lifting threads retain an impressive 90% of their original physical properties. Depending on the configuration and thickness of the selected VICTORIA lifting PDO thread, complete biodegradation occurs within approximately 420 days.

ADVANTAGES:
Uniform and consistent bioreabsorption over a longer period (up to 360 days);
Reliable and pronounced lifting result due to sequential bioreabsorption (the thread is securely fixed in the tissues, does not fragment or break down into separate elements, which reduces the risk of tearing in the tissues);
Physical properties of the polymer remain >90% up to 90 days post-implantation;
Placed in smooth, blunt-tipped atraumatic cannulas with triple polishing;
Shelf life—2 years from the date of manufacture.
Indications:
• Gravitational ptosis of soft tissues in the middle and lower thirds of the face;
• Middle third of the face — deep nasolabial folds, shaping and improving the facial contour, overall elevation of the lateral surface of the cheeks and zygomatic arch;
• Lower third of the face — «bulldog» cheeks in severe deformational ptosis, correction and restoration of the cervicomental angle;
• Defects in the submental area — double chin, deformation of the facial contour.
Legitimacy and safety:
VICTORIA PDO threads are approved by the FDA (U.S. FOOD & DRUG Administration). VICTORIA PDO threads are certified in the European Union (CE Marked) and in Eastern Europe (Ukraine, Russia, Kazakhstan, Armenia). Production facilities where VICTORIA® PDO threads are manufactured have KFDA, GMP, CE, ISO 13485/9001 certifications, which re-confirms the impeccable quality and it safety.
Disclaimer: Products on our website are intended for use by qualified healthcare professionals only. We are not responsible for any adverse effects from self-administration or for compensation of wasted or misused products. The information on this page is not a substitute for professional medical advice, diagnosis or treatment. Always consult a professional healthcare provider for a personalised medical guidance